X-chromosome inactivation research has emerged as a pivotal field in genetics, particularly due to its implications for understanding chromosomal disorders such as Fragile X Syndrome and Rett Syndrome. This intricate biological process, which allows females to effectively silence one of their two X chromosomes, has been under scrutiny for decades. At the forefront of this research is Jeannie Lee, whose innovative studies shed light on how cellular mechanisms orchestrate this silencing. The potential applications of her findings stretch beyond basic science, offering hope for gene therapy approaches that could treat X-linked disorders. As insights from X-chromosome inactivation continue to unfold, they promise not only to advance our understanding of genetic diseases but also to pave the way for groundbreaking therapies.
The study of X-chromosome silencing offers researchers a unique lens through which to view complex genetic phenomena. This process, which occurs in female cells to balance gene expression with their two X chromosomes, is crucial for unraveling conditions linked to genes located on the X chromosome, including prevalent disorders like Fragile X and Rett syndromes. By investigating the roles of specific RNA molecules and their interactions with chromosomal structures, scientists are opening the door to innovative treatments. Jeannie Lee’s work is instrumental in this area, demonstrating the potential for therapeutic advancements through the reactivation of silenced genes. As research progresses, the implications for gene therapy and the management of chromosomal disorders continue to expand, promising a brighter future for affected individuals.
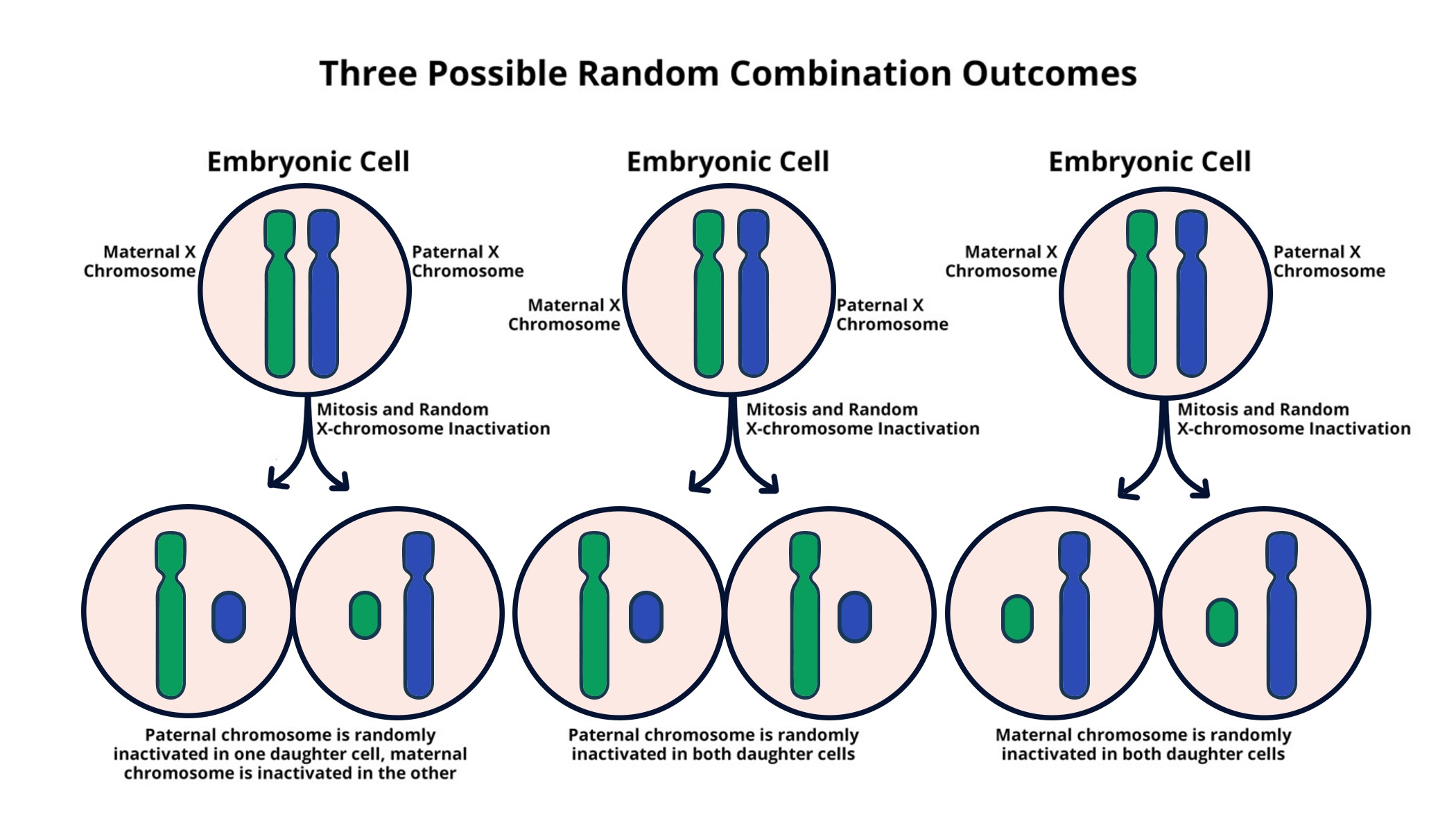
Understanding X-Chromosome Inactivation
X-chromosome inactivation is a crucial biological process that helps maintain genetic balance in females. This process ensures that with two X chromosomes present, only one is active while the other is silenced. Jeannie Lee’s groundbreaking research has illuminated how this silencing occurs, with the involvement of complex interactions between RNA molecules and surrounding chromosomal structures. By delving deep into the mechanics of this process, researchers can unlock potential therapeutic avenues to address disorders linked to X-linked mutations.
The implications of understanding X-chromosome inactivation extend beyond just basic biology; they open doors for developing gene therapy techniques aimed at treating various chromosomal disorders, including Fragile X Syndrome and Rett Syndrome. Gene therapy, which involves modifying genes to treat or prevent diseases, could benefit immensely from insights gained through studies in X-inactivation. As researchers like Lee unravel the precise mechanisms at play, it becomes increasingly possible to devise innovative treatments that might one day reverse the effects of genetic disorders.
Jeannie Lee’s Research Innovations
Jeannie Lee’s laboratory has pioneered research that reveals the intricate workings of X-chromosome inactivation. Her discovery that a gelatinous substance, likened to Jell-O, plays a crucial role in this process demonstrates how cells can manage the activation and silencing of chromosomes effectively. This finding is particularly significant not only for the field of genetics but also for its potential applications in treating conditions such as Fragile X Syndrome and Rett Syndrome. As the understanding of these mechanisms deepens, the potential for targeted therapies grows.
By employing innovative techniques to manipulate the X chromosome in cells, Lee’s lab is exploring ways to reactivate beneficial genes that have been silenced due to pathogenic mutations. This groundbreaking approach not only raises hopes for providing relief for patients suffering from conditions like fragile X syndrome but also enhances our overall understanding of neurodevelopmental disorders. The commitment to advancing research in gene therapy paves the way for future clinical trials that could potentially change the landscape of treatment for chromosomal disorders.
The Role of Gene Therapy in Treating Chromosomal Disorders
Gene therapy represents a revolutionary approach in treating chromosomal disorders, aiming to correct or replace dysfunctional genes responsible for diseases. With the insights gained from Lee’s work on X-chromosome inactivation, researchers are now on the frontline of exploring how to effectively deliver healthy genes to replace those that are mutated. This technique could have far-reaching impacts on diseases such as Fragile X Syndrome and Rett Syndrome, where the identification of the affected genes has long been established, but effective treatments had remained elusive.
As gene therapy techniques evolve, the positive results from initial studies in Lee’s lab can nurture hope for many families impacted by genetic disorders. By understanding the underpinnings of X-chromosome inactivation and innovating methods to target these genes directly, researchers are crafting a path toward not just managing symptoms but actually curing underlying genetic issues. It is a promising era for genetic research, auguring significant progress in combating the challenges presented by chromosomal disorders.
The Intersection of Chromosomal Biology and Therapeutics
The intersection of chromosomal biology and therapeutic development underscores the potential for groundbreaking discoveries to translate into real-world applications. The mechanistic insights provided by Jeannie Lee and her colleagues highlight how fundamental biological processes, such as X-chromosome inactivation, can inform the creation of targeted gene therapies. This intersection insists on the necessity of ongoing research to bridge the gap between basic science and medical innovation, particularly concerning neurodevelopmental disorders like Rett and Fragile X syndromes.
As scientists work to better understand how chromosomal structures influence gene activity, they are revealing avenues for therapeutic intervention that were previously unimaginable. Collaborative research, combining insights from chromosomal biology with advancements in technology and pharmaceutical development, will be essential in driving forward the next generation of gene therapies. By leveraging our understanding of phenomena like X-chromosome inactivation, researchers are equipped to tackle the complexities of genetic diseases that impact thousands of individuals worldwide.
Implications for Future Research in Genetics
The landscape of genetic research is rapidly evolving, with studies focused on X-chromosome inactivation paving the way for future exploration in genetics. The recent findings from Jeannie Lee’s lab provide a framework for understanding how gene expression can be modulated at the chromosomal level. These discoveries not only enhance our knowledge of genetic disorders but also set the stage for innovative findings that could lead to novel therapeutic strategies which are less invasive and more effective.
As researchers continue to decode the genetic underpinnings of diseases associated with the X chromosome, there lies a tremendous opportunity to correlate these findings with clinical outcomes. Future research initiatives will benefit from interdisciplinary approaches, integrating genetic sequencing, bioinformatics, and therapeutic modeling. By nurturing an environment that promotes collaboration, the scientific community can accelerate the pace of discovery and translate these breakthroughs into tangible improvements in patient care across various chromosomal disorders.
Understanding Fragile X and Rett Syndromes
Fragile X Syndrome and Rett Syndrome represent two critical areas of focus within genetic research, each linked to mutations on the X chromosome. Fragile X Syndrome is noted for causing a range of developmental issues, including intellectual disabilities, while Rett Syndrome affects brain development, leading to severe cognitive and physical impairments. Understanding how X-chromosome inactivation and other genetic mechanisms influence these conditions is crucial for developing effective treatments.
The recognition of the role that X-linked mutations play in these syndromes calls for tailored approaches in both research and therapeutic development. Insights from Jeannie Lee’s research can inform targeted treatments that aim to address the specific challenges posed by these disorders. As studies develop around gene therapy techniques and X-chromosome functionality, the hope is to create therapeutic options that significantly improve the quality of life for those affected.
Clinical Applications of Jeannie Lee’s Research
The clinical applications of Jeannie Lee’s research highlight a promising horizon for genetic therapies aimed at treating chromosomal disorders. By developing methods to unsilence genes that have been rendered inactive due to X-chromosome inactivation, her work paves the way for pilot clinical trials that could revolutionize treatment for patients with Fragile X Syndrome and Rett Syndrome. Such advancements could offer hope where previously there was little, potentially changing the lives of many families affected by these conditions.
The transition from laboratory research to clinical application is often fraught with challenges, yet Lee’s established methodologies are backed by decades of fundamental research. The ability to target specific genes for reactivation represents a substantial leap in the therapeutic landscape against chromosomal disorders. If successful, these approaches could usher in a new paradigm in which genetically engineered treatments are accessible, safe, and effective for a broad spectrum of patients.
Potential Challenges in X-Chromosome Inactivation Research
Despite the promising discoveries emerging from studies on X-chromosome inactivation, several challenges remain. One of the significant hurdles is accurately delivering therapies that can effectively target and unsilence the desired genes without affecting other healthy genes associated with the X chromosome. Lee’s research suggests that a delicate balance is required to maximize therapeutic efficacy while minimizing potential side effects, which is a critical aspect of developing any genetic therapy.
Additionally, the inherent complexities of chromosomal biology can create unforeseen obstacles as researchers navigate the intricate networks of gene expression. Understanding how other non-coding RNAs and chromosomal structures interact will be essential in refining therapeutic strategies aimed at disorders like Fragile X Syndrome and Rett Syndrome. Continuous research will be vital to overcoming these challenges, ensuring that the promises of gene therapy can be fully realized.
The Future of Genetic Research and Treatment
The future of genetic research is bright, particularly with breakthroughs in understanding X-chromosome inactivation. As researchers apply insights gained from studies conducted by scientists like Jeannie Lee, the promising horizon for genetic therapies becomes increasingly tangible. With advancements in technology and methodologies, the path toward effective treatments for chromosomal disorders is clearer than ever, offering hope to countless individuals and families.
Moreover, the ongoing collaboration between geneticists, clinicians, and biopharmaceutical companies will be pivotal in ensuring that research progresses effectively into clinical practice. As the field evolves and matures, the focus will be on translating foundational discoveries into viable treatment options. This confluence of basic research and clinical application signals a transformative era in the fight against genetic disorders, ensuring that patients suffering from conditions like Rett Syndrome and Fragile X Syndrome are no longer without options.
Frequently Asked Questions
What is X-chromosome inactivation and why is it important in genomic research?
X-chromosome inactivation is a biological process in females where one of the two X chromosomes is randomly silenced to prevent an overexpression of genes. Understanding this process is crucial in genomic research, especially regarding chromosomal disorders like Fragile X Syndrome and Rett Syndrome, as it could lead to potential gene therapy treatments.
How does Jeannie Lee’s research contribute to our understanding of X-chromosome inactivation?
Jeannie Lee’s research has significantly advanced our understanding of X-chromosome inactivation by elucidating the molecular mechanisms involved. Her lab identified how the RNA molecule Xist interacts with chromosomal material, guiding the silencing process, and opening new avenues for gene therapy in X-linked disorders.
What role does the substance similar to ‘Jell-O’ play in X-chromosome inactivation?
The ‘Jell-O-like’ substance, which coats chromosomes, aids in X-chromosome inactivation by creating a flexible environment that allows molecules like Xist to navigate and silence the X chromosome effectively. This gel-like barrier is essential for proper chromosomal organization and function during cell division.
Can X-chromosome inactivation research provide treatments for Fragile X Syndrome and Rett Syndrome?
Yes, research on X-chromosome inactivation is pivotal for developing therapies for Fragile X Syndrome and Rett Syndrome. By understanding the mechanisms that regulate X-chromosome activity, scientists like Jeannie Lee are exploring ways to unsilence healthy genes that are otherwise inactive, potentially offering new treatment options.
What are the potential clinical implications of unsilencing inactivated X chromosomes?
Unsilencing inactivated X chromosomes could lead to the restoration of function for mutated genes linked to X-linked disorders. Jeannie Lee’s findings suggest that this strategy may treat conditions like Fragile X Syndrome without significantly disturbing the function of healthy genes, offering a promising avenue for gene therapy.
How might gene therapy evolve from X-chromosome inactivation research?
Gene therapy may evolve from X-chromosome inactivation research by developing methods to target and unsilence specific genes on the X chromosome. This could allow for the reactivation of healthy genes that compensate for mutations causing disorders like Fragile X Syndrome, paving the way for effective treatments.
What are the challenges in researching X-chromosome inactivation mechanisms?
Researching X-chromosome inactivation mechanisms presents challenges such as the complexity of gene regulation and the cellular environment. Despite efforts over decades, the entire process is still not fully understood, particularly how unsilencing affects neighboring genes and overall gene stability.
| Key Points | Details |
|---|---|
| X-Chromosome Inactivation | Occurs in females to compensate for having two X chromosomes while males have one. |
| Role of Xist Gene | Xist RNA alters the biophysical properties of the Jell-O-like substance surrounding the X chromosome. |
| Nature of the Jell-O | Acts as a separator that prevents chromosome tangling and aids in X inactivation. |
| Therapeutic Potential | Unsilencing inactivated X chromosomes could lead to cures for diseases like Fragile X and Rett syndrome. |
| Current Research | The Lee lab is optimizing methods and moving towards clinical trials. |
| Unresolved Questions | Why inactivated X chromosomes can be freed without affecting other genes remains unclear. |
Summary
X-chromosome inactivation research has unveiled crucial insights into genetic regulation and treatment potentials. This significant breakthrough, led by experts like Jeannie T. Lee, not only clarifies the mechanisms of X inactivation but also opens doors for innovative therapies targeting genetic disorders linked to the X chromosome, such as Fragile X syndrome and Rett syndrome. As this research progresses, the hope is to translate these findings into actionable clinical therapies that could alleviate suffering for affected individuals.